Learn how blockchain truly works, master key definitions, and uncover what makes smart contracts so "smart." Dive into the fundamentals, gain valuable insights, and start your blockchain journey today!

- Analyst Corner
Andy Spinks
- on September 03, 2020
Why Blockchain can be a game changer for Pharma
The Patient is waiting
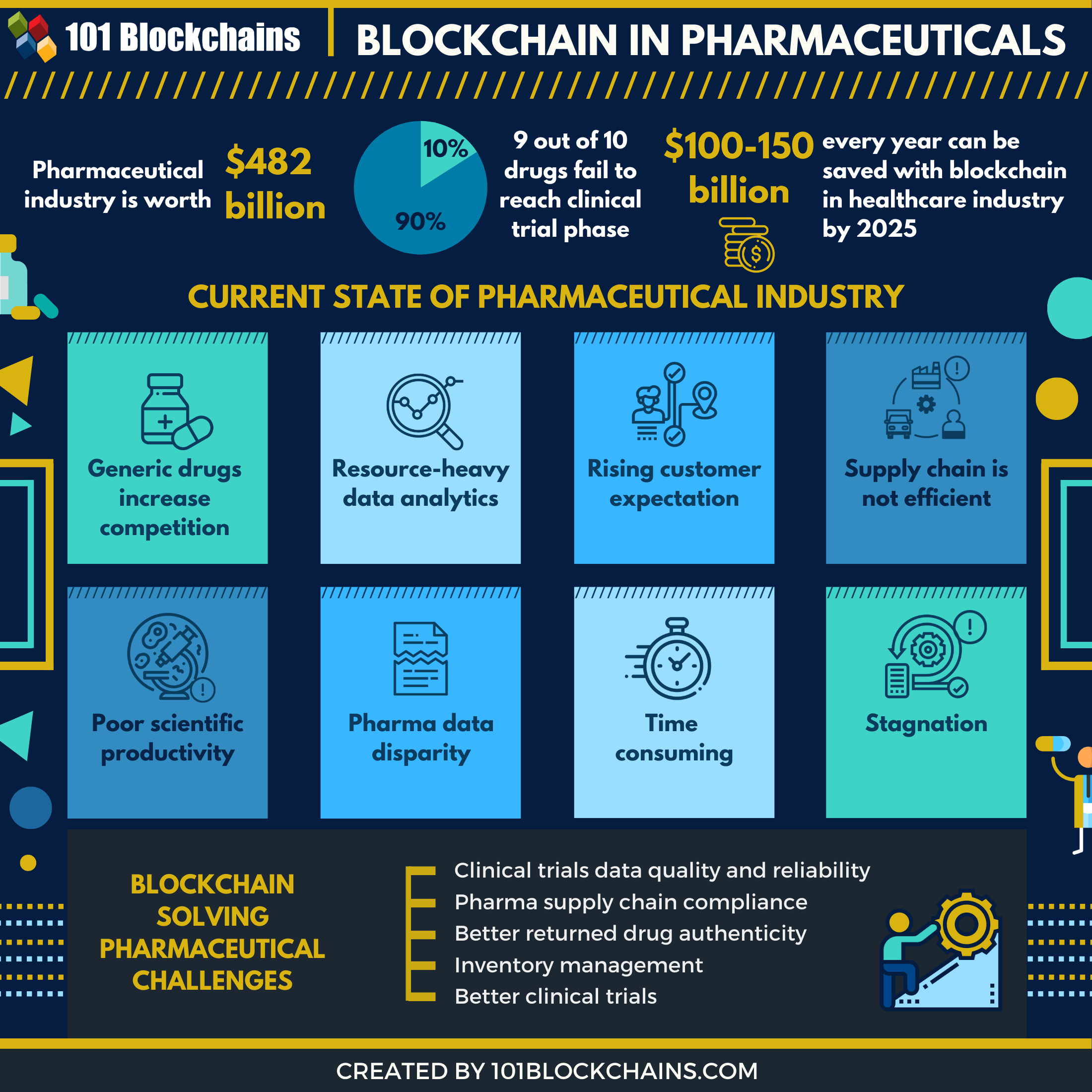
The Pharmaceutical industry’s primary goal is getting more effective drugs to patients quicker, its as simple as that. Or is it?
When you open the hood of a car, underneath there are a lot of moving parts which need to operate in unison. How well these parts collectively work to propel the car forward determines the speed you go and the gas you use.
The same principles apply to any Clinical Trial. A Pharmaceutical company will sponsor a study, often use one or more Clinical Research Organizations (CROs) to manage the study and a number of medical sites and Investigators will be contracted to recruit and manage the patients. In support of this you have all the enabling functions, drug supply, safety reporting, regulatory requirements and security needs to accommodate. A lot of moving parts and a lot of data. The more complex the study in terms of patients, sites, countries and partners the more data to control. When you start to expand this model across multiple studies within and across Pharmaceutical companies (and CROs) within different Therapeutic Areas and territories the growth in data is exponential.
Can you trust your data?
Historically, to manage this, study data is often duplicated, copied and stored multiple times in a number of systems for a number of companies. This is not intentional but often a result of circumstances such as:
- A lack of integration between disparate existing applications
- Years of acquisitions and mergers creating many legacy systems
- Software customizations and in-house work arounds
- Inconsistent and out of date processes
Whatever the reason this can create major issues when trying to co-ordinate, report and correlate data leading to extensive rework, manual verification and review. This in turn invariably leads to study delays and higher costs.
And whilst time to market for drugs is obviously important to waiting patients it is also critical to drug manufacturers and not just because it cost more the longer a study takes (which it does).
The clock is ticking
Most people may be aware that drug patents in the US are for 20 years. What many may not know, however, is that the moment a drug patent is filed and successfully registered, the clock starts ticking on that 20 years. If a drug then takes 10 years to go through the trial phases before getting approved and to market then that patent only has 10 years left. That is the real cost implication and part of the reason that drugs can appear so expensive as the manufacturer often has a limited time to recoup their huge R&D costs before the patent expires.
It is estimated that Pharma companies lose $600K in potential revenue for every day a trial is delayed for a niche drug, $8M per day for a blockbuster. According to the Clinical trials.gov website there are currently over 350,000 trials running around the world.
Blockchain as a data enabler
It’s ability to address these challenges is why Blockchain can be a game changer for Pharma.
What if individual study data could be in one secure, controlled location with a full built in audit trail. What if common shared industry data could be stored in single source of truth repositories accessed by all those who need it and updated via a structured consensus model. What if patients could own their personal data in a single place. What if Pharma companies could focus on competitive advantage rather than each addressing industry wide common issues. All of these would save significant time and money individually and collectively for Pharma companies and improve the experience for study teams and patients. This is what Blockchain can enable!
Based on this model, even if only a few percent of these trials make it to market, if Blockchain saves just one day per study through improving time to recruit patients, pay investigators, monitor sites or improve compliance that would justify its cost several times over. However, applied appropriately, Blockchain has the potential to drive significantly greater gains. Remember cheaper, quicker studies also potentially opens the way to more affordable drugs in patients hands sooner.
Right time, right place
Historically Pharma companies have focused on their own needs and goals which is understandable but, from an industry perspective, this has left potential gaps in seeking to address common issues and improvement opportunities which would benefit all. Although this requires upfront investment and collaboration, if done properly, it would then subsequently free up company resources to focus on the real value add activities.
This is why Pharma needs to take notice of blockchain, it can be a game changing enabler for the industry and should not be seen as just another technology. It is not replacing existing technologies but, used appropriately, can complement and enhance them creating a smoother, quicker flow of data and information.
Also Read: Blockchain In Pharma
Learn more
In this video 101 Blockchains highlights some of the areas where blockchain could help the Pharma industry move forward together and speed up the clinical trial process dramatically. But if blockchain is to have the impact it could in this industry it also requires a new collective mindset from all those involved.